Abstract
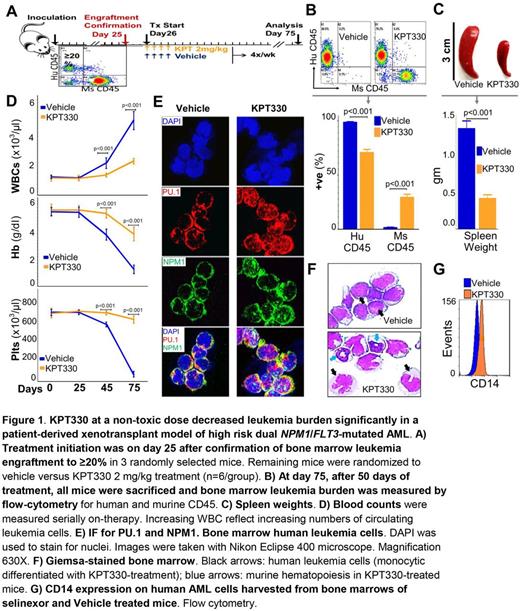
NPM1 is the most frequently mutated gene in acute myeloid leukemia (AML). Unfortunately, there are no 'precision' or rational treatments for this subtype of AML.To elucidate molecular mechanisms of pathogenesis, we performed the first comprehensive, unbiased analysis of the endogenous NPM1 protein-interactome using mass-spectrometry (LC-MS/MS). This approach identified abundant amounts of the master transcription factor driver of monocyte lineage-differentiation PU.1 (SPI1). The NPM1/PU.1 interaction causes PU.1 functional deficiency when NPM1 is mutated, because mutant-NPM1 dislocates PU.1 into the cytoplasm with it.This was confirmed using six different methods: (i) Immunoprecipitation (IP)-LC-MS/MS from nuclear and cytoplasmic fractions of wildtype (wt) and NPM1 -mutated AML cell lines (n=2); (ii) IP-Western blot (WB) from nuclear/cytoplasmic fractions of NPM1 -mutated/wt AML cell lines (n=2); (iii) WB of nuclear/cytoplasmic fractions of NPM1 -mutated/wt AML cell lines (n=5); (iv) immunofluorescence microscopy (IF) of NPM1 -mutated/wt AML cell lines (n=5); (v) IF of NPM1 -mutated/wt primary AML cells (n=6); and (vi) cotransfection of HEK293 cells to express PU.1 + mutant vs wt-NPM1 followed by IF. Re-introduction of Pu.1 into the nucleus of Pu.1-null myeloid precursors which are differentiation-arrested and exponentially proliferating repressed key myeloid precursor genes (e.g., Hoxa9) and triggered terminal monocytic differentiation. Even though primary AML cells (n=900) express the PU.1/RUNX1/CEBPA monocyte differentiation-driving master transcription factor circuit at levels comparable to or exceeding that in normal monocytes, the expression of ~300 monocyte terminal-differentiation genes was suppressed. Importantly, the genes affected are strongly positively correlated (avg. rho 0.7) with PU.1 expression in normal hematopoiesis, consistent with functional disruption of PU.1 in AML. To translate these observations into a treatment option for NPM1 -mutated AMLs, we were guided by additional observations. First, the NPM1/PU.1 protein complex is exported by the nuclear export protein XPO1. XPO1-mediated nuclear export is inhibited by the small molecule selinexor. We found that sub-cytotoxic /low nanomolar concentrations of selinexor locked mutant-NPM1/PU.1 in the nucleus, releasing terminal monocytic differentiation of NPM1 -mutated AML cells both in vitro and in vivo . Briefly, NSG mice were xenotransplanted with NPM1 / FLT3 -mutated primary AML cells and observed until AML engraftment (≥20%) was confirmed. Selinexor was then administered by oral gavage at 2 mg/kg 4X/week (Fig1A), which is 10-fold lower than the usual in vivo cytotoxic dose (15-20 mg/kg) - low doses are well-tolerated and sufficient to promote non-cytotoxic differentiation of AML cells. After 50 days of treatment, bone marrow (Fig1B) and spleen (Fig1C) AML burden was significantly lower in selinexor vs vehicle treated mice. In addition, selinexor treated mice preserved murine hematopoiesis (Fig1D) and IF confirmed the partial nuclear restoration of PU.1 (Fig1E). Terminal monocytic differentiation of AML cells was evident by Giemsa-stained morphology (Fig1F) and flow cytometry (Fig1G). RUNX1 and CEBPA remain in NPM1 -mutated AML cell nuclei at high levels - PU.1 usually cooperates with these master transcription factor partners to exchange corepressors for coactivators and activate differentiation genes. Accordingly, IP-LC-MS/MS of endogenous nuclear CEBPA demonstrated enrichment for corepressors. Depletion of one of these corepressors, DNMT1, using non-cytotoxic concentrations of decitabine or 5-azacytidine (clinical DNMT1-depletors), also induced terminal-differentiation. Moreover, the granulocytic direction of differentiation naturally downregulated NPM1, an event inherent to CEBPA-driven granulocytic (but not monocytic) differentiation. This approach readily induced terminal-differentiation in NPM1 -mutated AML cells selected over 52 weeks of culture for resistance to selinexor, shown by exponential growth in selinexor 20 nM. The mechanisms by which mutant-NPM1 creates leukemic self-replication (proliferation uncoupled from differentiation) are thus reversed by non-cytotoxic molecular targeted clinical drugs. We are evaluating the combination of selinexor with non-cytotoxic DNMT1-depletion in vivo and clinical trials are planned.
Landesman: Karyopharm Therapeutics: Employment.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal